About us
IDMIT charts and organigrams
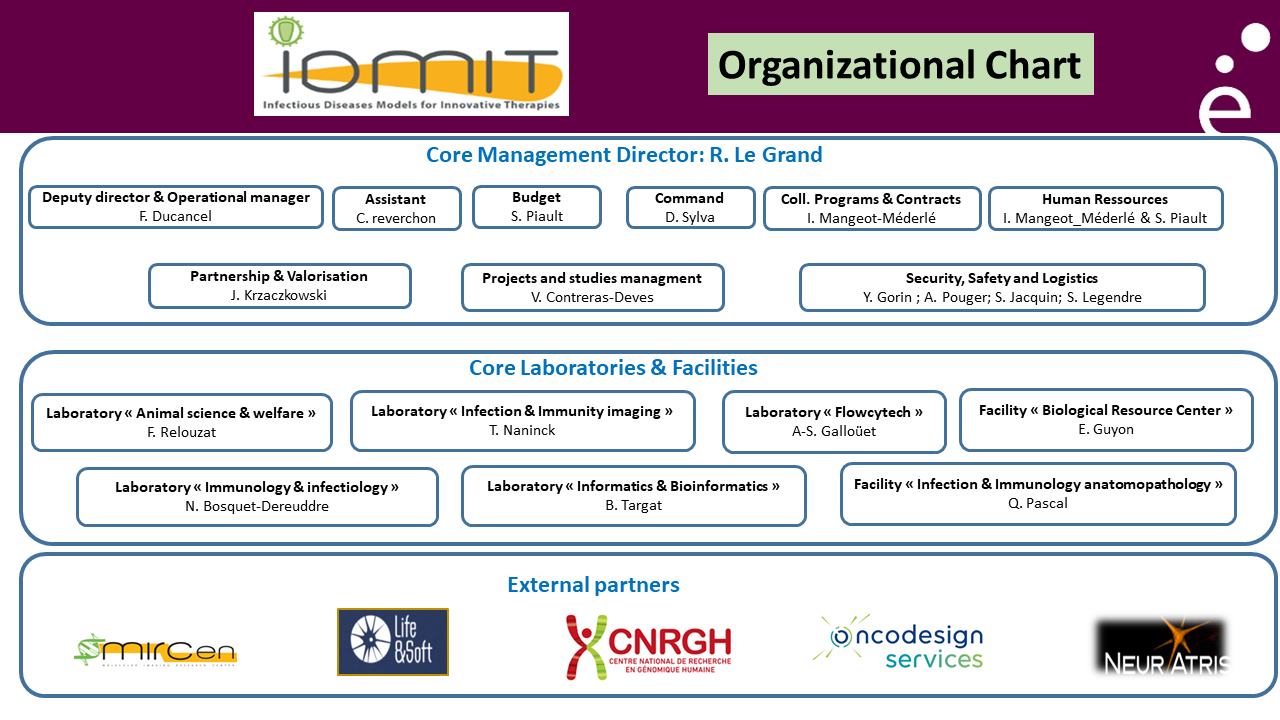
IDMIT “Organization” chart
IDMIT national infrastructure is composed of the following entities:
– a “Core management” group (CEA-Fontenay-aux-Roses)
– a “Core laboratories & facilities” entity (CEA-Fontenay-aux-Roses)
– an “External partners” consortium
The Management system of the national Infrastructure IDMIT was successfully certified in 2017 (27th of July and renewed in 2020 and 2023) by LRQA (Lloyd’s Register Quality Assurance Limited) to the International Quality Management System Standard ISO 9001:2015.
The scope of this approval is applicable to: “Research and services for the evaluation of new therapeutic approaches in particular for the treatment of human infectious diseases”.
The general objective is to provide the best research, technological development, environment or service to our partners/customers whether internal, external, public, private, national or international.
Overall Quality Policy that we wish to implement.
IDMIT “Governance” chart
The overall IDMIT Governance is ensured by two boards and two committees.
The board of Directors, headed by Pr. Françoise Barré-Sinoussi (Nobel Prize in Medicine 2008) is composed of the directors of the six co-founder partners:
– Institut Pasteur: Pr. Stewart Cole
– CEA-DRF: Dr. Anne-Isabelle Etienvre
– ANRS: Pr. Yazdan Yazdanpanah
– INSERM: Dr. Didier Samuel
– University Paris Saclay: Pr. Estelle Iacona
– Oncodesign Services: Dr. Fabrice Viviani
Its missions are:
– to define the general development strategy for the IDMIT Center
– to nominate the Executive director of IDMIT (nomination for 5 years)
– to approve the strategy for IDMIT Center organization and management proposed by the Executive Director
– to approve the annual scientific, managerial and financial reports supplied by the Executive Director
– to define rules for prioritization of access to IDMIT Center facilities, core laboratories and services.
The Scientific Advisory Board, is composed of eight internationally recognized experts in the field of infectious diseases. They are mandated for 3 years:
– Dr. Jan Langermans (BPRC, Netherlands)
– Dr. Jeff Lifson (NIH, USA)
– Pr. Luis Montaner (The Wistar Institute, USA)
– Pr. Pierre Delobel (CHU Purpan, France)
– Pr. Camille Locht (Institut Pasteur de Lille, France)
– Pr. Paul Johnson (Emory University, USA)
– Pr. Galit Alter (Harvard Medical School, Moderna USA)
– Pr. Stefan Treue (German Primate Center, Germany)
The IDMIT steering committee, is composed of representatives of the six co-founder partners:
– Dr. Roger Le Grand (CEA)
– Dr. Michaela Müller-Trutwin (Institut Pasteur)
– Dr. Valérie Thibaudeau and Dr. Evelyne Jouvin-Marche (Inserm)
– Dr. Françoise Bachelerie (University Paris-Saclay)
– Dr. Cécile Peltekian (ANRS MIE)
Its main mission is to assist the Excutive Director (Dr. R. Le Grand) in the management of IDMIT infrastructure:
– IDMIT investment choices in agreement with the functioning of the different core facilities
– evaluation of IDMIT submitted projects feasibility
– prioritization of IDMIT infrastructure access
– IDMIT communication towards the scientific community
– exchanges place between the different partners
The IDMIT ethics committee is directly linked to Ethics Committe of the Life Science Division of the CEA (CETEA-CEA DSV IdF), created and registered (6th of June, 2011) by the French Ministry of Higher Education and Research. As such, IDMIT Ethics Committee, follows the governance of the “Charte nationale portant sur l’expérimentation animale” according to the EU-Directive_2010_63 on the protection of animals used for scientific purposes.
IDMIT Ethics Committee is composed of veterinary doctors, researcher and zoo technician representatives and of external personalities.
Its overall mission is to give his opinions on any experimental project implying vertebrate animals. It analyses the scientific objective of the animal experimentation phase of the project, to decree about the ethic acceptability of the animal model choice linked to the proposed experimental protocol and methodology.
IDMIT proposes a large set of core facilities that allow an exhaustive exploration of the genomic, transcriptomic, molecular, cellular and tissular parameters, necessary for preclinical studies and validations in Non Human Primates (NHP) animal models of innovative preventive and/or therapeutic solutions. Among them one can cite the cutting-hedge technologies CyTof and whole NHP body optical and nuclear in vivo imaging. In addition, a Biological Resource Center was created, when bioinformatic tools are developed to ensure analysis and modelization of IDMIT-generated data.